
AIKYA PHARMA
STAYING AHEAD OF PHARMACEUTICAL MANUFACTURING TRENDS
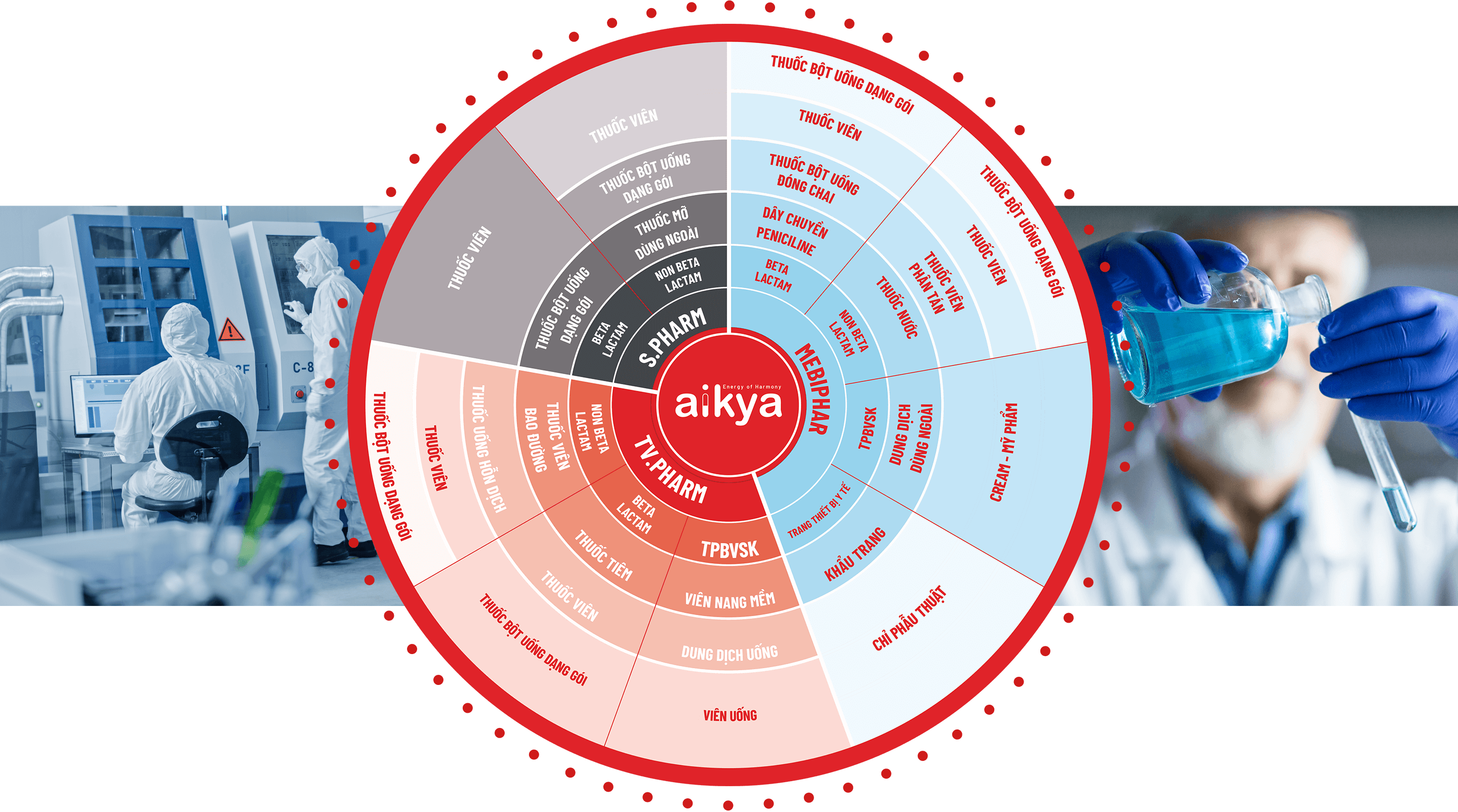
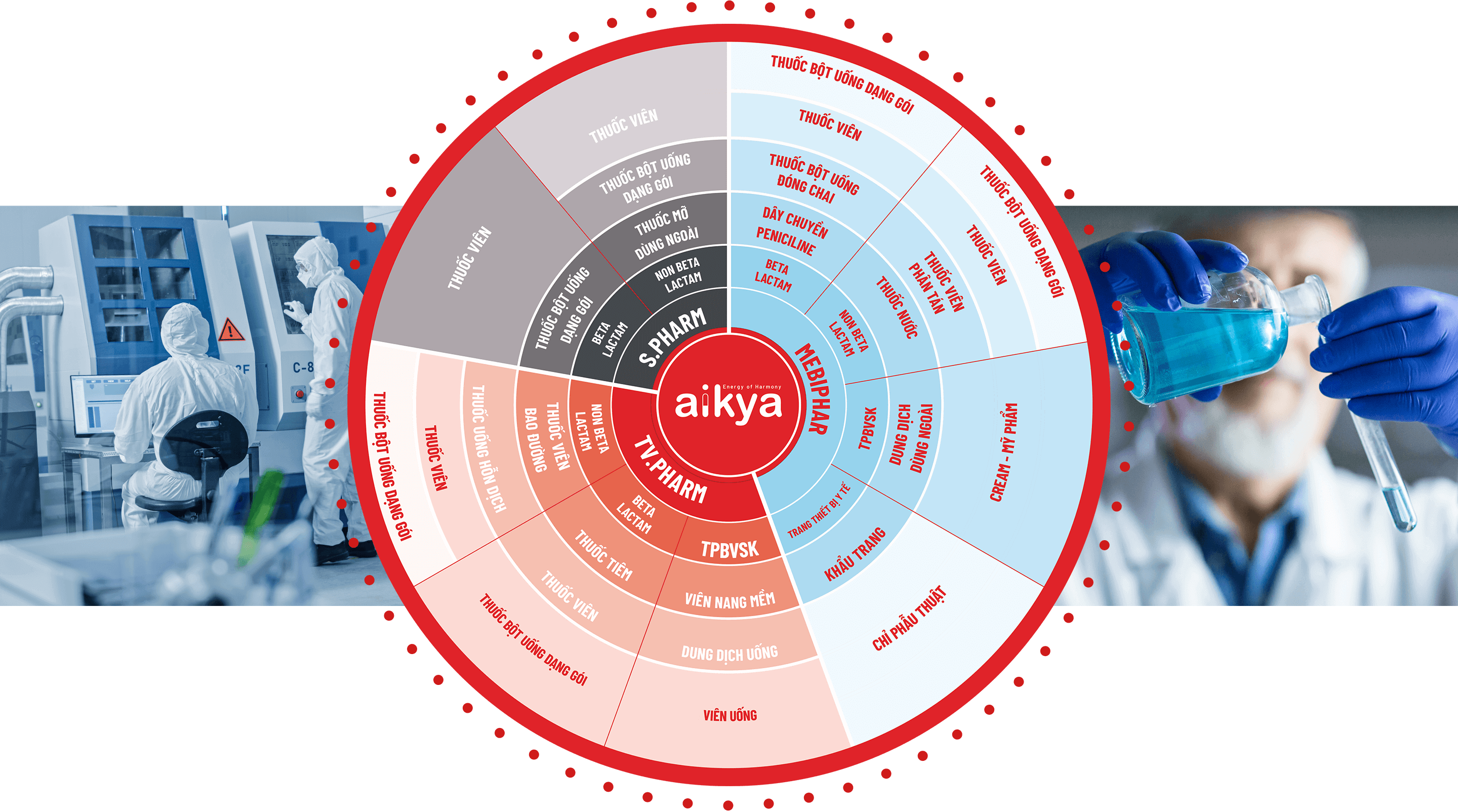
Aikya ranks among the top 10 generic drug manufacturers in Viet- nam, offering a diverse product portfolio that spans various thera- peutic areas to meet broad medical needs. Aikya is proud to own three highly reputable pharmaceutical companies in Vietnam:
- TV.PHARM Pharmaceutical Joint Stock Company
- S.PHARM Pharmaceutical Joint Stock Company
- Medical Biomaterial & Pharmaceutical Joint Stock Company (MEBIPHAR)
Our manufacturing facilities are adaptable to changes in product volume and order timelines. All our production lines fully comply with WHO-GMP standards and are set to be upgraded to EU-GMP standards by 2024, reaffirming Aikya’s commitment to maintaining the highest quality levels for its products..
Currently, Aikya operates over 20 modern production lines, including tablets, powders, liquids, injections, cosmetics, and health supplements.
CURRENT PRODUCTION
CAPACITY:







ESTABLISHING
THE LARGEST PHARMACEUTICAL INDUSTRIAL CLUSTER IN VIETNAM
Vietnam’s pharmaceutical industry is a highly promising market, not only in Asia but also with the potential to expand globally. Many domestic pharmaceutical companies have been investing in upgrading their facilities, increasing production capacity, and enhancing competitiveness against imported products. EU-GMP (Good Manufacturing Practice) stands as one of the highest quality standards in the global integration trend that all Vietnamese pharmaceutical companies aim to achieve.
Recognizing these immense growth opportunities, Aikya has boldly invested in the construction of the high-tech pharmaceutical industrial cluster TV.PHARM, with the vision of not only meeting the healthcare needs of the domestic population but also aspiring to bring Vietnamese pharmaceutical products to the international market.
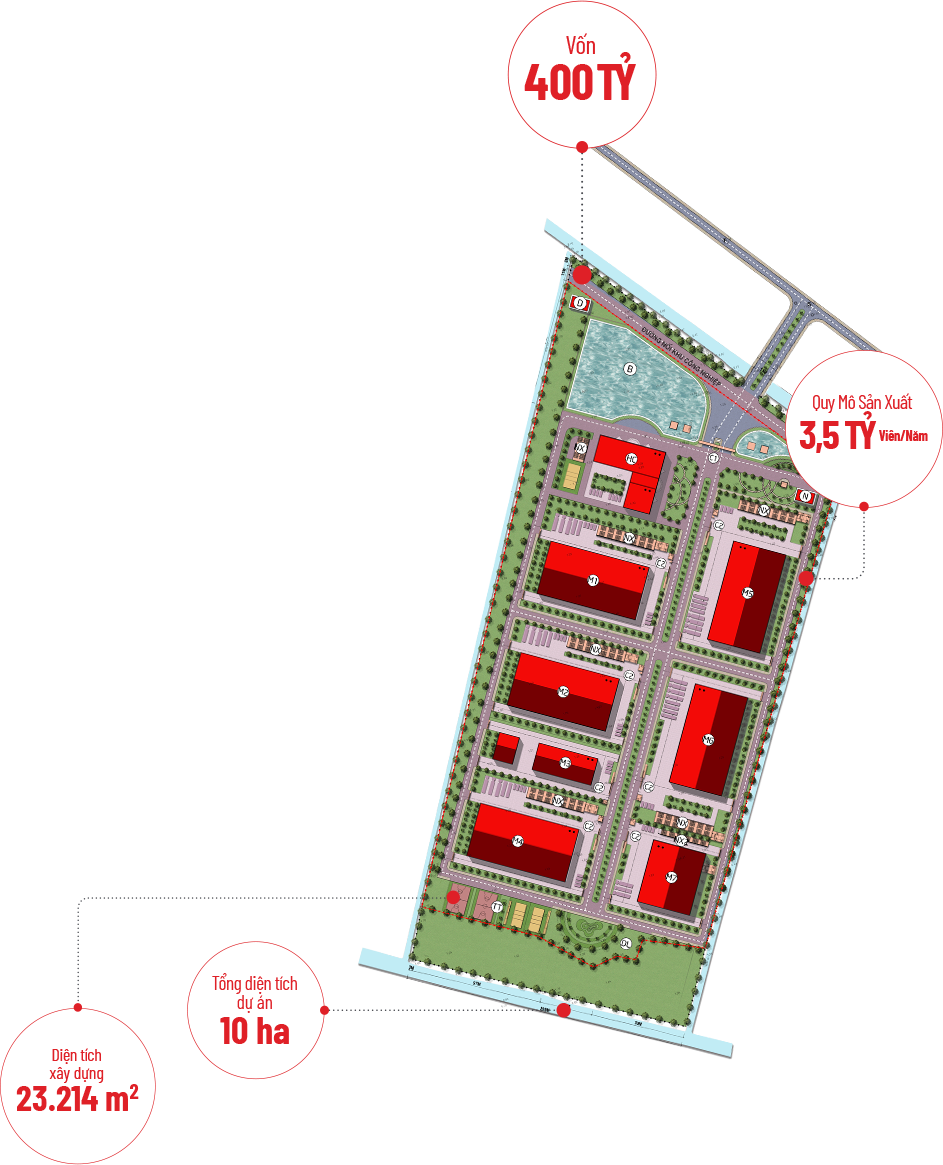
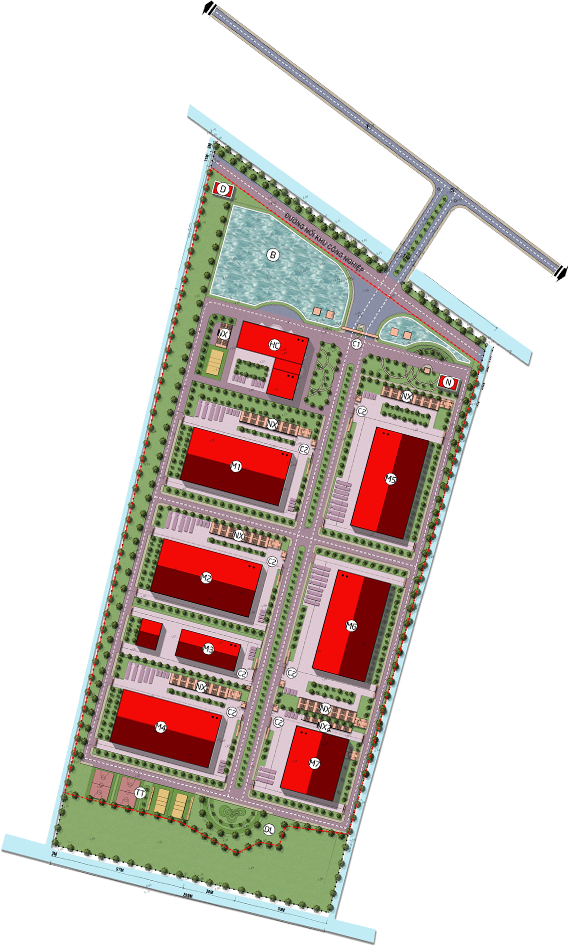
In 2020, Aikya commenced the construction of Vietnam’s largest pharmaceuti- cal industrial cluster project in Tan Ngai hamlet, Luong Hoa A commune, Chau Thanh district, Tra Vinh province, featuring.
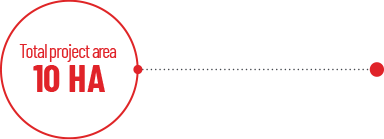
- A total project area of nearly 10 HECTARES, with a construction area of 23,214 M2
- An investment capital of 400 BILLION VND
- Production facilities MEETING EU-GMP, FDA, PICS, and WHO STANDARDS
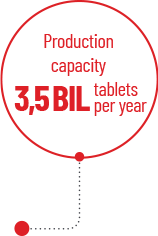
- A projected production capacity of 3.5 BILLION TABLETS PER YEAR, capable of meeting domestic treatment needs and export demands
With this ambitious project, Aikya is committed to its mission of ensuring that every product reaching customers meets the highest standards. We continuously strive to strengthen our leadership position in Vietnam’s pharmaceutical industry while expanding our influence globally.


FACTORY
AIKYA EUROPA
In 2022, Aikya began the construction of its first facility within the High – Tech Pharmaceutical Industrial Cluster – the AIKYA EUROPA factory. As the flagship project of the TV.Pharm pharmaceutical cluster, the factory specializes in non-beta-lactam tablet production, including tablets, film-coated tablets, and hard capsules. The facility spans nearly 6,000 square meters across two floors, encompassing warehouse areas, production workshops, a quality assurance laboratory, technical rooms, and office spaces, all integrated into a unified structure.
All products at AIKYA EUROPA are manufactured on fully automated production lines with advanced technology, covering the entire process from raw materials to tablet pressing, capsule filling, and blister packaging, resulting in a finished product. The machinery adheres to CFR 21 Part 11 Standards, and the software for each machine complies with GAMP 5 and COPA-Data guidelines, providing solutions that ensure safety and security in environments regulated by the U.S. FDA. With a rigorous quality control system, all materials and products meet EU GMP standards and comply with Good Manufacturing Practice guidelines. The AIKYA EUROPA factory will produce products that adhere to the highest quality and reliability standards.

To enhance credibility, all products manufactured at AIKYA EUROPA will be encoded with unique identification numbers, allowing for full traceability of the TV.Pharm manufacturer worldwide, ensuring top-level quality assurance and anti-counterfeiting measures.
In addition to its high-tech infrastructure and international standards, AIKYA EUROPA is operated by a young, highly skilled team of engineers and pharmacists who stay updated with the latest EU GMP regulations and international expert guidance. Once fully operational, the factory is expected to produce 600 million units annually, encompassing over 100 products in categories such as cardiovascular, diabetes, pain relief, and digestive-cerebral circulation medications. The products are set to be officially exported to the European market, SRA countries, and many other regions globally.


